1.Good safety of gelatin-free.The first lyophilized Varicella Vaccine, containing no gelatin from animals, invented and produced in China. Getting rid of gelatin from varicella vaccine can significantly decrease the ratio of anaphylaxis incidence .
2.Long validity period by good stability. The first approved varicella vaccine with 36 months of validity period in the world. Adopting BH-2 stabilizer with own IP rights (Chinese patent granted No.: ZL200910138411.6, International patent application No.: PCT/CN2009/001405) greatly enhances the stability of the product and ensures the validity period for 36 months under the storage condition.
3.Better protection with high titer and immune eff Varicella Vaccine(Live) Live Bioproducts Pharmaceutical,Live High Potency Medicine,Live Preventive Pharmaceutical,Varicella Biopharmaceutical Live Changchun BCHT Biotechnology Co.,Ltd , https://www.ccbcht.net
At the site of the first phase project of the comprehensive utilization of 60,000 tons/year of carbon dioxide gas from the COSCO Gas Co., Ltd. in Quangang District, the company’s general manager, Yu Weixi, excitedly told reporters.
Quangang District cooperates with the first phase of the comprehensive utilization of carbon dioxide waste gas, with a total investment of 50 million yuan. It applies the most advanced CO2 professional treatment technology in China, mainly through the recovery of CO2 tail gas emitted from the refinery process of Fujian United Petroleum & Chemical Co., Ltd., through desulfurization and pressure-swing adsorption. , Gas separation, purification, refinement and cryogenic liquefaction produce food-grade liquid carbon dioxide with a purity of 99.99%. The project is expected to be put into production in October this year, and the annual production capacity will reach 60,000 tons. It will provide high-quality carbon dioxide products to the major shipbuilding companies, construction machinery, automobile manufacturing, food and beverage industries in coastal areas of Fujian Province. The new production value of 35 million yuan, tax more than 700 million. At the same time, the project can eliminate 30 million cubic meters of carbon dioxide gas in one year, which will play a unique role in promoting the construction of a national-level circular economy demonstration pilot park in Quangang.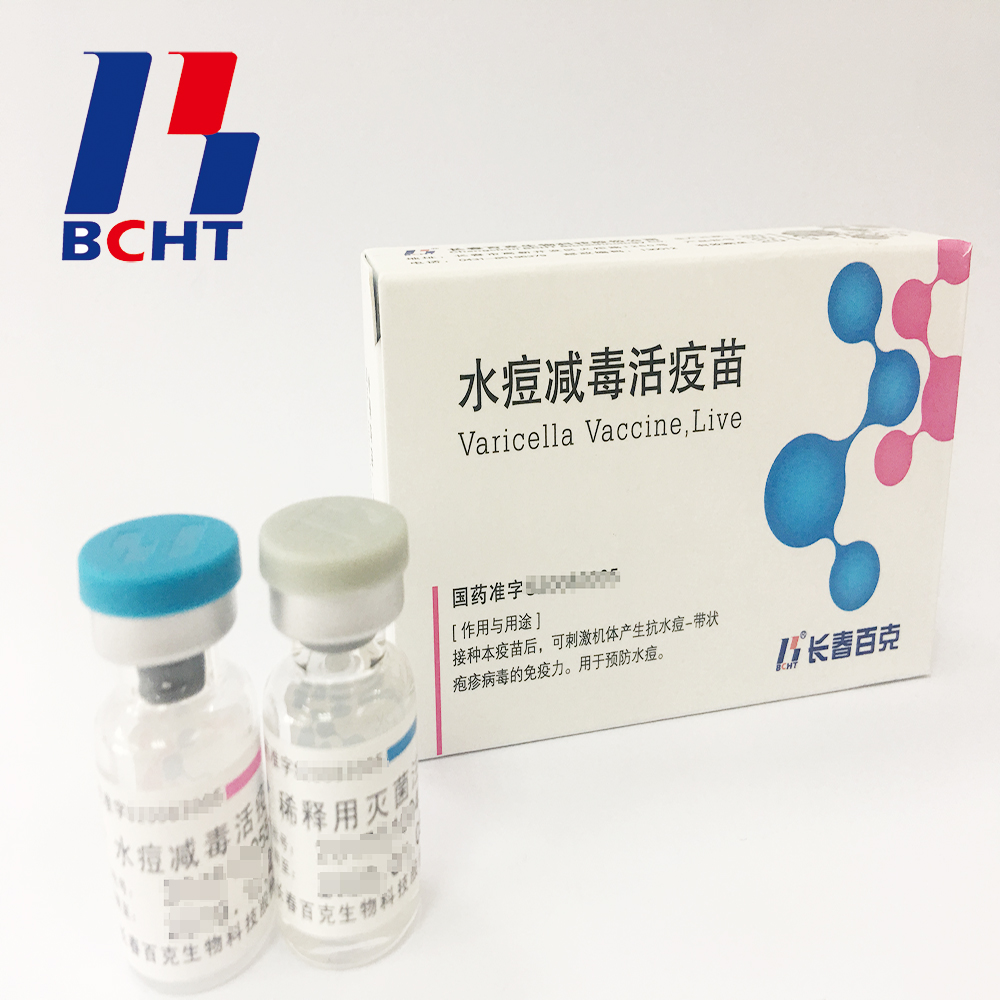 icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
Fujian's largest carbon dioxide refining project put into operation
At present, the largest CO2 refinement project in Fujian Province has entered the trial production stage, and it has successfully produced industrial-grade carbon dioxide products with a purity of 99.95%. It is rushing to produce food-grade carbon dioxide products with a purity of 99.99%.