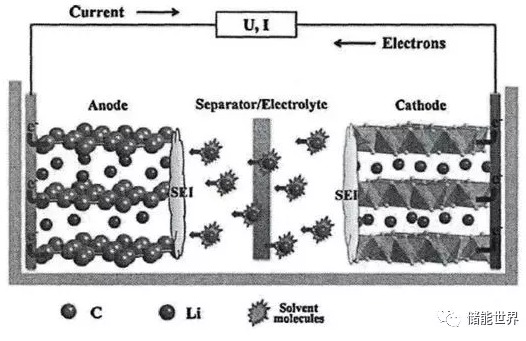
As the nickel content increases, the stability of the positive electrode material decreases. The main manifestation is the capacity loss of cyclic charge and discharge and the accelerated attenuation of high temperature environment capacity. Recently, the news of the power lithium battery technology route, the introduction of high-nickel ternary lithium battery will become the main force of the power battery in the next few years, the energy density stepped on the 300Wh / kg step. This article aims to be onlookers and care about the past and present of high-nickel ternary materials. 1. Lithium battery working principle Currently common lithium batteries, mainly ternary lithium, lithium iron phosphate, lithium manganate, lithium cobalt oxide, etc., are named according to the type of cathode material. The commercial anode materials used in conjunction therewith are generally graphite anodes. The basic working principle is shown in the figure below. As shown in FIG. During the charging process, due to the external voltage of the battery, the electrons in the vicinity of the positive current collector move to the negative electrode under the electric field. After reaching the negative electrode, they combine with the lithium ions in the negative electrode material to form a local electrical neutral storage in the graphite gap. The surface of the negative electrode that consumes a part of lithium ions has a low lithium ion concentration, and a difference in ion concentration is formed between the positive electrode and the negative electrode. Under the concentration drive, the lithium ions in the positive electrode material move from the inside of the material to the surface of the positive electrode, and along the electrolyte, pass through the separator to the surface of the negative electrode; further, under the action of the potential, diffuse into the deep material of the negative electrode, and The electrons coming from the external circuit meet, and the local display is electrically neutral and stays inside the negative electrode material. The discharge process is just the opposite. After the circuit containing the load is closed, the discharge process begins with electrons flowing out of the negative current collector and through the external circuit to the positive electrode; finally, lithium ions are embedded in the positive electrode material and combined with the electrons coming from the external circuit. The negative graphite is a layered structure, and the manner in which lithium ions are intercalated and extracted is not much different in different types of lithium ions. There are significant differences in the lattice structure of different cathode materials, and the lithium ions diffuse in and out during charge and discharge, and the process is slightly different. 2. Types and characteristics of main cathode materials The cathode materials which are currently fully commercialized are mainly lithium cobaltate, lithium iron phosphate, lithium manganate and ternary lithium. Among them, lithium cobalt oxide has obvious advantages in terms of energy density, etc., but the safety problem has become a bottleneck, and the scope of use is getting smaller and smaller. Lithium manganate has poor cycle performance and poor high temperature performance. Although it has strong anti-overcharge capability and low cost, it is mainly used only on low-end or low-speed vehicles, and its market share is also shrinking. Only lithium iron phosphate and ternary lithium are the current mainstream, one of them has the advantage of energy density and low temperature performance, and the other has the advantages of cycle life and safety, national policy and end users in between Some are difficult to decide. So far, the bus mainly uses lithium iron phosphate, and passenger cars and other models that require higher battery life and customer experience require a ternary lithium battery. 3. Structure and characteristics of ternary lithium cathode material Ternary materials are the hotspots of the past few years. Among them, the Ni component can improve the activity of materials and increase the energy density. The Co component is also an active material, which can stabilize the layered structure of the material, reduce the cation mixing and facilitate the deep discharge of materials. , thereby increasing the discharge capacity of the material; the Mn component acts as a support in the material to provide stability during charging and discharging. Ternary lithium basically reflects the advantages of several materials. Under the large category of ternary materials, the ratio of the three metal elements in the material is different and can be regarded as different kinds of ternary materials. One type is Ni:Mn isometric type, and the second type is Ni:Mn isometric type. Representatives of the isotypes are NCM424 and NCM111. During the charge and discharge process, the +4 valence Mn constant price plays a role in stabilizing the structure. The +2 valence of Ni becomes +4 valence, losing two electrons, making the material have a high specific capacity. Ni, Mn is not the same type, is the protagonist of this article, also known as high nickel ternary lithium, the main representative models are NCM523, NCM622 and NCM811. When the nickel-rich ternary material is lower than 4.4 V (relative to Li+/Li) on the voltage platform, it is generally considered that Ni is +2/+3 and participates in the redox reaction, and the valence increases to +4. When the voltage is higher than 4.4 V, Co3+ participates in the reaction to become +4 valence, and Mn4+ does not participate in the reaction to stabilize the structure. The effect of high nickel ternary on the positive electrode Different ratios of NCM materials have different advantages and can be selected according to specific application requirements. Ni exhibits high capacity and low safety; Co exhibits high cost and high stability; Mn exhibits high safety and low cost. In order to improve the energy density of the battery and increase the driving range of the vehicle, the current mainstream view is to improve the safety of high-nickel ternary in the high-nickel direction to meet the vehicle use requirements. In addition to the mature commercial technology routes such as ternary and lithium iron phosphate, lithium manganate and lithium cobalt oxide mentioned above, there are also many technical directions such as lithium-sulfur batteries, lithium-air batteries and all-solid-state batteries, but they are all mature. Commercial is still far away. The electrochemical properties and safety of ternary lithium batteries depend mainly on the microstructure (particle morphology and volume structure stability). And the influence of physicochemical properties (Li+ diffusion coefficient, electron conductivity, volume expansion ratio, and chemical stability). Ni increases the cycle performance; thermal stability deteriorates; surface reaction is uneven during charge and discharge; a large proportion of Ni2+ is present in the reaction product, resulting in oxidation of the material, slow oxidation of the electrolyte, and gas evolution during the process. 4. High nickel cycle performance problems As the nickel content increases, the stability of the positive electrode material decreases. The main manifestation is the capacity loss of cyclic charge and discharge and the accelerated attenuation of high temperature environment capacity. 4.1 Capacity attenuation mechanism in the cycle The capacity attenuation factors in the cycle mainly include cation mixing, stress-induced microcrack generation, introduction of impurities in the production process, redistribution of conductive carbon black, etc., in which the capacity is attenuated by two factors: cation mixing and microcrack generation. The role is most significant. The cation mixing and discharging means that the volume of the divalent Ni ion itself is similar to that of the lithium ion, and when the lithium ion is largely desorbed during discharge, it is subjected to external factors and occupies a position in the Li ion lattice. The dislocation of the ions brings about a change in the type of crystal lattice, and the lithium insertion ability also changes. During the charging and discharging process, the surface of the positive electrode material is decompressed with the highest pressure and the fastest, so the surface often changes the surface lattice due to the cation mixing. This phenomenon is called surface reconstruction. The higher the Ni content, the higher the probability that the trivalent unstable Ni ions are reduced to divalent Ni ions, and the more opportunities for cation mixing occurs. The other two metals, Mn and Co, have a much smaller ratio than Ni. Inhibition of cation mixing, the researchers mainly consider the following points: 1) Take measures to reduce the formation of divalent Ni ions, and fundamentally cut off the root cause of the mixing; 2) Doping Mg ions with a volume close to that of the divalent Ni ions, the Mg ions can preempt the vacancies left by Li earlier than Ni, and avoid the entry of Ni. Mg ions are not directly involved in the charge and discharge process, and can be stabilized in position after embedding to support the material structure. 3) Adjusting the molar ratio of Ni to Li in the raw material of the positive electrode material and adjusting the preparation process to reduce the influence of the raw material on the cation mixing. Impurities are introduced during the production process, and in the preparation of the positive electrode raw material, reaction with air water and Co2, etc., produces a material type that does not exist originally, such as lithium carbonate. When there is a large amount of Li2CO3 on the surface of the material, gas is decomposed during the cycle, and the surface adsorbed on the surface of the material causes poor contact between the active material and the electrolyte, and the polarization is increased, and the cycle performance is also deteriorated. Microcrack During the charging and discharging process, the volume of the positive electrode material changes. The higher the Ni content, the larger the volume expansion ratio. The generation of cracks also depends on the magnitude of the charge-discharge cut-off potential. Therefore, the operating voltage of the high-nickel layered oxide positive electrode (relative to the lithium metal negative electrode) is usually not more than 4.1 V. The purpose is to ensure that irreversible phase transition does not occur and the inner diameter is reduced. stress. The cracks on the crystal and the separation between the crystals make the positive grain of the high-nickel ternary material inevitably subject to a larger volume variable. During the process of volumetric cycle variation, cracks may occur between the grain boundaries inside the primary grain, and the distance between the grains and the grains will gradually increase, and some grains will separate from the positive electrode. More crystal faces are in contact with the electrolyte to form more SEI film, which consumes electrolyte and active material while increasing the resistance of lithium ions to diffusion on the electrodes. Attenuating the phase transition tendency in the cell voltage range is a method of suppressing microcracks. The current main directions of researchers are as follows. 1) inhibiting the doping of magnesium ions in the cation mixing, including the lattice of magnesium ions, the direction of expansion is substantially uniform, and can play the role of suppressing microcracks; 2) The NCM811 material is prepared as a two-phase composite material uniformly embedded in the Li2MnO3 structural unit, which can reduce the volume change. Redistribution of conductive materials This influencing factor is mainly about NCA, and NCM has not published relevant research. After a certain cycle of circulation, the conductive material is redistributed on the surface of the crystal grain, or a part of it is separated from the crystal of the active material, which causes the dynamic environment of each part of the crystal to become different, thereby causing crystal cracking. The further effect after the occurrence of the crack is consistent with that described in the previous "microcrack". 4.2 High-temperature environmental capacity accelerated attenuation mechanism After a certain period of high temperature cycle, it is found that there are a large number of inactive bivalent and trivalent Ni ions between the grain boundaries, and the Ni ions exiting the cycle cannot participate in the charge compensation. The battery capacity attenuation ratio is similar to the amount of the deactivated ions. It is speculated that the main form of capacity decay under the high temperature and low voltage window is caused by the inactivation of Ni ions. In addition, the high temperature cycle easily causes the lattice of the positive electrode material to collapse, and changes from NiO6 to NiO, thereby losing activity. Tests have shown that the poor conductivity of the SEI film will also cause high temperature cycle capacity attenuation. In the context of the pursuit of overall performance beyond the traditional fuel vehicles, the pursuit of energy density can be said to be a hot spot for power lithium batteries for more than ten years. At the same time, the security issue is the threshold for the large-scale commercialization of batteries. Advances in other devices in the power battery pack, such as battery management systems, such as various sensors, can also make up for some of the battery safety in the process. Prefab Office,Modular Office,Prefab Flatpack Camp,Modular Flatpack Camp Yangzhou Tonglee Reefer Container Co., Ltd. , https://www.cimctlc.com